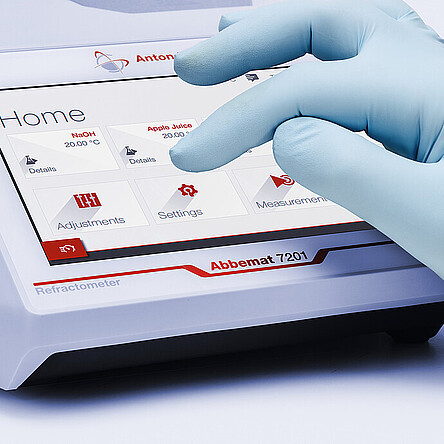
Pharmaceutical Refractometer:
Abbemat Pharma 7001
The pharmaceutical refractometers are designed to exceed the requirements of pharma regulations and those of other regulated environments: 21 CFR Part 11, GAMP 5, and EU GMP Vol. 4 Annex 11. They provide secure data management, audit trails, electronic signatures, and access controls, ensuring audit readiness at all times, as well as integration into processes and secure storage of measuring and metadata. The AISQ+ qualification package speeds up validation by 70 %, offering risk analysis, SOPs, and traceability documentation for quick deployment in regulated environments
Key features
Comprehensive qualification for your pharmaceutical refractometer
The performance qualification and routine monitoring of measuring equipment go beyond just verifying the refractive index. Anton Paar also ensures the accuracy of a crucial second parameter: temperature.
With the patented T-Check system, Anton Paar service engineers validate precise temperature measurements, offering:
- On-site, traceable temperature verification and adjustment
- Highly accurate prism-surface temperature measurement
- Prevention of temperature-related measurement errors
- Comprehensive documentation and certification
Preventing Errors Through User Guidance
User rights management ensures that operators have access only to the functions necessary for their role. Changes to settings are restricted to users with the appropriate privileges. User profiles can be seamlessly integrated from Active Directory.
Routine measurements can be structured according to your SOP with predefined pass/fail criteria. Check intervals can be configured to enforce system suitability tests in accordance with your measuring equipment control protocols.
Secure data management options
The automatic refractometers can be used stand-alone with file-based data export to your network. Alternatively, data can be managed by Anton Paar Connect: An encrypted SQL database that is not susceptible to manipulation ensures safe data storage, straightforward backup and restore, and easy accessibility of data throughout the retention period.
Digital AISQ+ qualification package
Comprehensive analytical instrument and system qualification (AISQ+) greatly reduces the effort to go live with your new pharmaceutical refractometer. It is available printed or fully digital with electronic signatures, speeding up review and approval in the qualification process.
Durable Sapphire Prism – Scratch-Resistant with 25-Year Warranty
Engineered for durability, the premium optical components of your automatic pharmaceutical refractometer guarantee exceptional resilience and a long lifespan.
- Withstands cleaning with spatulas and other laboratory tools
- Features a Mohs hardness of 9, nearly diamond-grade
- Comes with a 25-year warranty on the prism